Gilles Billen, Sorbonne Université
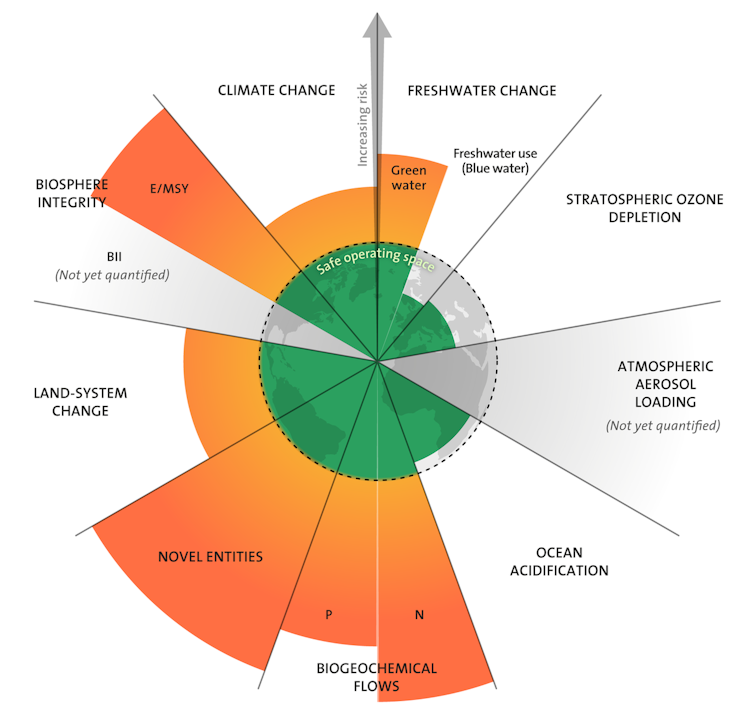
Six of our nine planetary boundaries have been crossed– industrial agriculture is the main culprit. That is what a team of scientists under Johan Rockström reported in an article published in September.
First, a reminder: planetary boundaries are thresholds of disturbance beyond which our Earth’s system is put on an uncontrollable and irreversible path that undermines the very conditions for life. This notion of overstepping boundaries is clear regarding the best-known limit of them all: climate change. In order to cap global warming at 1.5°C above pre-industrial levels and prevent it from escalating beyond bearable conditions (even if this is not enough to stop climate disruption already underway), we need to keep the proportion of atmospheric CO₂ below a certain limit. And to achieve this, we need to reach carbon neutrality, quickly.
Regarding the climate, it is easy to see how such a global limit is relevant: the carbon cycle is open to the whole planet, and CO₂ emitted (or captured) anywhere on Earth immediately affects the world’s atmosphere in its entirety. Yet, in the case of the planetary boundary for nitrogen, exceeding the threshold is different, as agriculture’s industrialization is largely, and more complexly, responsible for breaking the limit.
But how can agriculture affect the nitrogen cycle? How has it managed to reach a breaking point? To feed the world, isn’t intensive agriculture best? Let’s take stock of the situation.
The natural nitrogen cycle
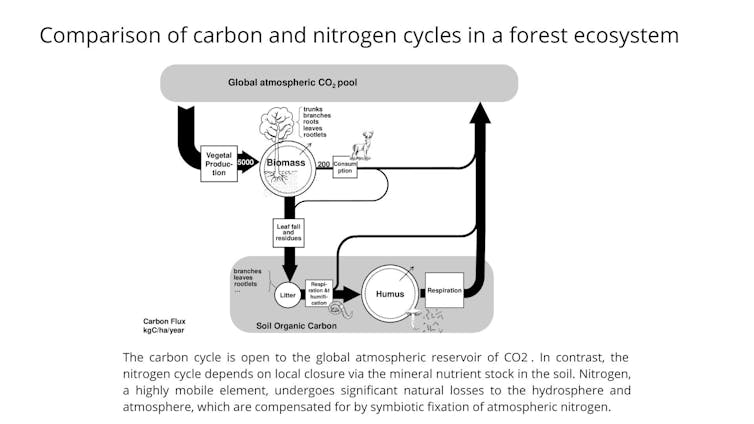
First, we need to understand the natural cycle of carbon and nitrogen – two of the main elements that form living matter. We can observe this cycle in forests, for example. To function, forests rely on a balance between plant growth – a process that turns mineral (inorganic) forms of carbon and nitrogen into biomass (organic) – and animals, fungi, and micro-organisms decomposing this biomass, a process that remineralizes it.
But whereas the inorganic form of carbon (CO₂) is present in the atmosphere, distributed evenly worldwide, and absorbed by plants via their leaves, nitrogen is remineralized in the soil and absorbed by plant roots. So, the boundaries of the nitrogen cycle have to remain local: any loss of nitrogen brings about a risk of soil depletion, which jeopardizes continued plant growth.
Gilles Billen, Author provided (no reuse)
Yet, inorganic nitrogen in the soil is remarkably mobile. It exists in several forms, including ammonia (NH₃, NH₄+), which is very volatile; nitrous oxide (N₂O), which is gaseous; and nitrate (NO₃-), which is highly soluble. The amount of nitrogen that is lost in the atmosphere and in groundwater is, therefore, considerable, and this loss makes nitrogen the main limiting factor in plant growth.
However, nitrogen is widely present in the Earth’s atmosphere, making up 78%. But it exists in the atmosphere in its molecular form as N₂ – an inert gas that most organisms are unable to use. Only plants in one particular family – legumes (peas, lentils, beans, clover, alfalfa, and some trees, such as acacia) – are able to draw on this reserve of gaseous nitrogen. They do so through a symbiotic association with bacteria that have enzymes needed to convert molecular nitrogen into proteins. This symbiotic fixation offsets the natural environmental loss of nitrogen and ensures that terrestrial ecosystems function perennially.
Farming and fertilization
In agricultural systems, the nitrogen cycle is structurally open. Each time plants are harvested, the nitrogen contained in them is carried far away from the plot of soil where it came from. So, to avoid soil depletion, nitrogen taken away from the soil – whether through harvesting or environmental loss – has to be put back in the soil in one way or another. That is the purpose of fertilization.
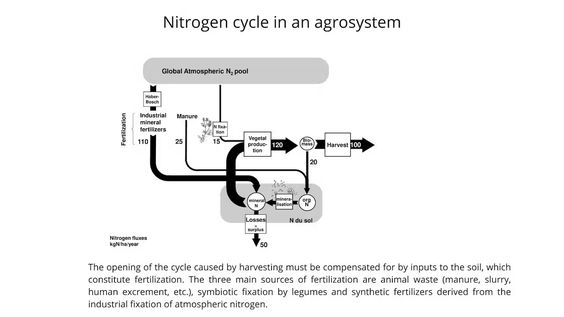
There are many methods of fertilization. Excrement from animals and humans that have eaten plants can be spread as fertilizer. That is the most natural process for keeping the nitrogen cycle closed. However, it can be hard to carry out properly if the place where the plants are eaten is a long way from the place where they are cultivated. However, the excrement of livestock grazing on semi-natural pastures can be spread on neighboring arable land. This process transfers the fertility of these pastures to the neighboring arable land.
Indeed, this method was the basis of traditional systems of polyculture and livestock farming. Another way of providing cereals with nitrogen is crop rotation: growing cereals in alternation with leguminous plants on the same plot. With this method, nitrogen is fixed by the leguminous plants that grew on the plot before the cereals did.
Add to that mix industrial fertilisers, which have been around for just over a century. Shortly before the First World War, the German chemists Fritz Haber and Carl Bosch developed a process for producing ammonia (NH₃) and, later, nitric acid by using high temperatures and pressures to force a reaction between nitrogen from the air and hydrogen (from coal back then, but today from natural gas).
This process was first used to make explosives, but it later served to mass-produce synthetic nitrogenous fertilizers. These new fertilizers increasingly became the go-to option to fertilize farmland soil. They quickly made traditional polyculture and livestock farming obsolete and paved the way to intensified and specialized agriculture, which was henceforth coupled with the heavy chemical industry.
Some writers consider the Haber–Bosch process to be “the most important industrial process” of modern history, foreshadowing, among other inventions, the airplane, nuclear energy, or the television. In 1925, the biologist Alfred Lotka marveled
This extraordinary development the Haber–Bosch process is something more than a fundamental new departure in industry. It represents nothing less than the ushering in of a new ethnological era in the history of the human race, a new cosmic epoch.’
And that is what this issue is about: this new epoch that we call the Anthropocene. Today, on a global scale, the quantity of reactive nitrogen that the fertilizer industry puts into the biosphere each year exceeds the amount provided by all natural processes of biological fixation. On a global scale, the speed at which nitrogen circulates has increased more than twofold.
Environmental nitrogen loss
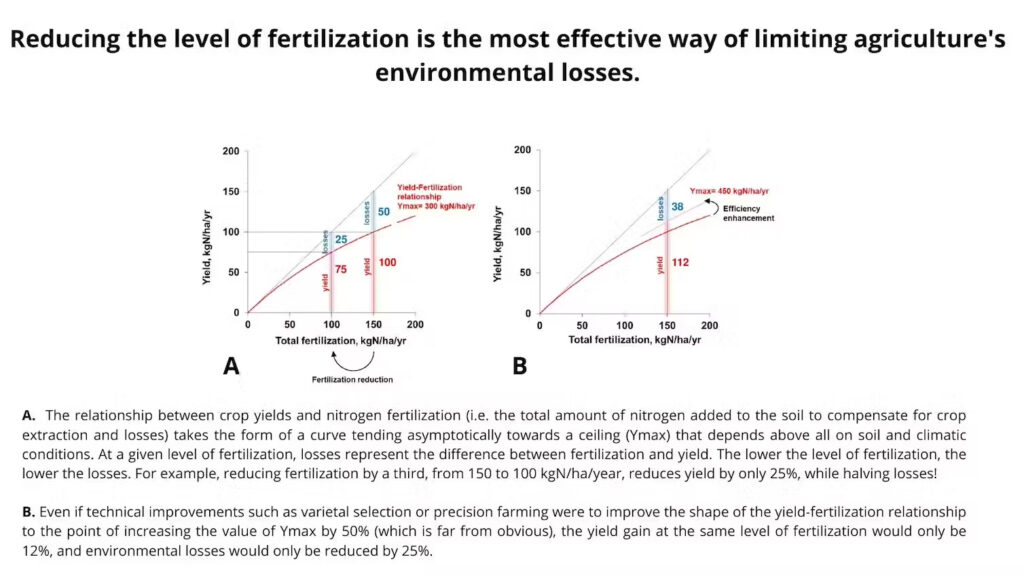
In this accelerated flow of nitrogen, what causes trouble is the environmental nitrogen loss that results from it. Indeed, the more nitrogenous fertilizers are used to increase crop yields, the less the added nitrogen is effective and the greater the losses through leaching and volatilization. What we call the nitrogen surplus is the excess of nitrogen put into the soil in relation to the quantity actually taken away through harvesting.
This surplus contaminates groundwater, making it undrinkable, and river water, which leads to the eutrophication of coastal waters – the cause of green tides, toxic algal blooms, and deep-sea anoxia.
And it is this surplus that releases ammonia into the atmosphere, which creates aerosols with serious effects on human health..
That is why the team working under Rockström evaluated the agricultural nitrogen surplus when defining the planetary boundaries beyond which the conditions for human life on Earth would no longer be guaranteed. The upper limit of this surplus, which is determined to protect water and air locally, varies greatly between world regions, but on a global scale, it is estimated to be 60 million tonnes of nitrogen (60 TgN/year), in contrast to today’s nitrogen surplus of around 130 TgN/year.
This huge gap between the threshold not to be overstepped and the actual level reached today justifies the goal that the European Commission and the United Nations Biodiversity Conference recently set to halve nitrogen waste by 2030.
Yet it is not by simply adjusting practices that nitrogen waste from agriculture will be halved so that the planetary boundaries are respected. Industrial producers of fertilizers promote the progress offered by precision agriculture, nitrification inhibitors applied to soil, varietal improvement of crops, and so forth.
These new methods that promise progress might open up lucrative markets for the agricultural supplies industry, but everything else points to them bringing only a negligible drop in nitrogen waste. Indeed, the most effective way to boost efficiency and reduce loss is to scale down agricultural production itself!
Feeding the world without ruining it
But can we reasonably scale down intensive farming without jeopardizing the food security of a world that will have 10 billion mouths to feed by 2050? Many recent studies claim that we can. Yet, we can only do so if three major structural changes are made to the entire agrifood system while intensive agriculture is toned down.
The first change needed is to make widespread use of crop systems that have proved their worth in organic farming, based on long-term and diversified crop rotation where cereals are grown in alternation with leguminous plants. With this approach, both synthetic fertilizers and pesticides can be abandoned.

The second change needed is to reconnect crops with livestock farming, where the farm animals are only fed local fodder (grass, leguminous fodder, and grain only when it can be produced in excess of human needs) and where their excrement can be reused on the same site. This ensures that the nitrogen cycle remains as closed as possible.
With this approach, industrial livestock farming can be abandoned, making it possible to reduce the proportion of animal products in human diets substantially. This is the third change needed. A diet where meat and dairy products are reduced to 30% of people’s total intake of proteins (in contrast to 65% in France currently) would not only be healthier, insofar as it would reduce the risk of cardiovascular diseases and certain cancers, but it would be fairer too, insofar as it would reduce the share of agricultural production that is currently dedicated to feeding livestock. Furthermore, this approach could be widespread for all humans throughout the globe. On a European scale, it has been shown that only this agroecological scenario would effectively halve nitrogen waste and greenhouse gas emissions from agriculture.
So, we need to stop assuming that the only way to meet the planet’s growing needs in food is the continued intensification of industrial agriculture, continued specialization in agriculture, and continued growth in the international trade of agricultural products. On the contrary, this model of agriculture has now been clearly identified as a factor that disturbs the Earth’s system profoundly. We will only be able to feed tomorrow’s world while respecting the conditions for life on Earth by making major structural changes to the global agri-food system based on frugality, reconnection, and agroecology.
This article is part of a project between The Conversation France and AFP Audio, supported financially by the European Journalism Center, as part of the Bill and Melinda Gates Foundation’s “Solutions Journalism Accelerator” initiative. AFP and The Conversation France have maintained their editorial independence at every stage of the project.![]()
Gilles Billen, Directeur de recherche CNRS émérite, biogéochimie territoriale, Sorbonne Université
This article is republished from The Conversation under a Creative Commons license. Read the original article.


1 Comment
Pingback: Our children and grandchildren must wonder what we were thinking of. And with. - Bergensia